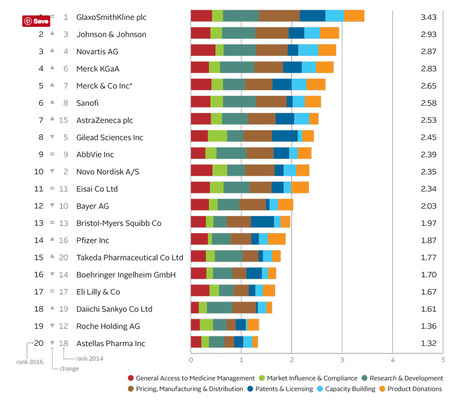
The Access to Medicine Index analyses the top 20 research-based pharmaceutical companies on how they make medicines, vaccines and diagnostics more accessible in low- and middle-income countries.
In 2016 moderate progress is visible in the pharmaceutical industry’s efforts to improve access to medicine. GSK leads for the fifth time, ahead of Johnson & Johnson, Novartis and Merck KGaA.
Leaders see business rationale in access
GSK leads for the fifth time ahead of Johnson & Johnson, Novartis and Merck KGaA. Critically, these companies show needs-orientation, matching actions to externally identified priorities in the access agenda. For example, they invest in R&D for urgently needed products, even where commercial incentives are lacking. Their access strategies support commercial objectives, with clear business rationales.
Incremental improvements
Lower ranked companies have each improved in at least one measure, and withstood closer scrutiny: the 2016 Index used tougher measures than in 2014. Change by these companies has been incremental. Exceptions are Takeda, which launched a new access strategy and rose from 20th place, and Bayer, which lost ground as others improved.
In the top ten
Following the first four, the remaining companies in the top ten each show strength in at least one area, yet have room to deepen engagement in access to medicine. There have been two significant shifts in this group. Novo Nordisk falls to 10th place. Its solid access frame- work applies to few products (albeit those considered key for access). AstraZeneca joins the top ten, with an expanded access strategy and notable pricing practices.
Lowest rankings
Lagging furthest behind are Roche** and Astellas. Roche is less transparent than its peers, yet it advances in other measures, with new access initiatives and strong processes for ensuring compliance. While Astellas shows some improvements, such as a new pledge not to enforce IP rights in certain poor countries, these were not sufficient to avoid being overtaken.
- Best practices
- Company records Cards
- Key findings
etc.
Via rob halkes

 Your new post is loading...
Your new post is loading...









Just recently the Access to Medicine Index 2016 for Pharma Companies has been published!
See all and the download here